Dave Summary
go back to main Dave Earl page
David J. Earl
Theoretical predictions of molecular chirality and chirality transfer
The concept of chirality is simple: if the mirror image of an object is non-superimposable upon the original object then the object is said to be chiral. An example of this is a right-hand whose mirror image is a non-superimposable left-hand. In the same way that hands are chiral, so too are some molecules. Chiral molecules have an enormous range of uses in a wide variety of fields.
A very interesting property of chiral molecules is that when they are added to an achiral (non-chiral) liquid crystal solvent they can transmit their molecular chirality to the whole system over distances many times their molecular length, giving the liquid crystal a right-handed or left-handed (depending on the handedness of the chiral molecules added) helical twist. Moreover, different chiral molecules can exert different levels of twist to a system (a property known as the helical twisting power). The addition of different chiral molecules to an achiral solvent can result in completely different helical pitches. Clearly some molecules are more chiral than others. Chiral molecules with very high helical twisting powers are of great industrial importance. They are used in liquid crystal displays and in chiral polymer films. The synthesis of new chiral dopant molecules can be a long and costly process, and the helical twisting power of the new molecule is unknown until it can be determined by experiment after it has been synthesised. Clearly a theoretical model that could predict helical twisting powers would be a very useful tool in determining what molecules to synthesise in the future.
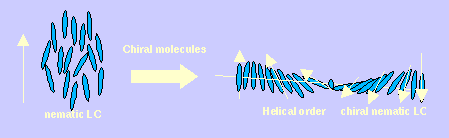
My research has focussed on developing models / theories that can be used to make theoretical predictions of the helical twisting powers of chiral molecules. Two main approaches have been used:
1) This approach directly measures the impact of a chiral molecule on the liquid crystal solvent. Using statistical mechanics, it is possible to relate free energy changes and intermolecular torques to the helical twisting power. This approach requires modelling of the chiral molecule at an atomistic level and of the liquid crystal solvent at a generic level. My first project was to develop a liquid crystal solvent for use in these simulations. My research on soft-repulsive spherocylinders [1] was the first detailed study on this class of potential and two liquid crystalline phases were found in its phase diagram. I then utilised this solvent to calculate the helical twisting powers of five chiral molecules. This was done by gradually inserting the chiral molecule into the liquid crystal solvent over the course of a simulation and measuring the free energy change for this process. Good agreement with experimental results was found and the handedness of twist produced by the molecules was correctly predicted in all cases [2]. I have also recently conducted work on measuring intermolecular torques. It is possible to relate the torque the chiral molecule induces in the solvent, as chirality is transferred to the system, to the helical twisting power [3].
2) I have also worked on single molecule techniques that can rapidly be used to predict the helical twisting power of a new molecule. These methods, based on a scaled chiral index [4,5] and a chirality order parameter [6], have been incorporated into a new Monte Carlo simulation program that can be used to screen molecules for high helical twisting power values prior to synthesis. This program has been used to study how different molecular conformations can result in different helical twisting powers [5] and to investigate the temperature dependence of the helical twisting power [7].
The work I have conducted on molecular chirality has provided new insight into the properties of chiral molecules. A number of powerful techniques now exist that provide a quantitative description of molecular chirality. These descriptions can now be directly linked with experimentally determined properties such as the helical twisting power. The systems I have studied are also of great importance in liquid crystal displays and my research has been sponsored by an industrial company for these purposes (Merck NB-C).
References
[1] D. J. Earl, J. Ilnytskyi and M. R. Wilson, “Computer simulations of soft repulsive spherocylinders”, Mol. Phys.99, 1719 (2001)[2] M. R. Wilson and D. J. Earl, “Calculating the helical twisting power of chiral dopants”, J. Mater. Chem.11, 2672 (2001)[3] Calculations of helical twisting powers from intermolecular torques. D. J. Earl and M. R. Wilson, J. Chem. Phys., 120, 9679-9683 (2004).[4] M. Solymosi, R. J. Low, M. Grayson, M. P. Neal, M. R. Wilson and D. J. Earl, “Scaled chiral indices for ferroelectric liquid crystals”, Ferroelectrics277, 483 (2002)
[5] M. P. Neal, M. Solymosi, M. R. Wilson and D. J. Earl, “Helical twisting power and scaled chiral indices”, submitted to J. Chem. Phys. (2003)
[6] A. Ferrarini, G. J. Moro, P. L. Nordio and G. R. Luckhurst, “A shape model for molecular ordering in nematics”, Mol. Phys. 77, 1 (1992)
[7] D. J. Earl and M. R. Wilson, Predictions of molecular chirality and helical twisting powers: A theoretical study. J. Chem. Phys., 119, 10280-10288 (2003).
